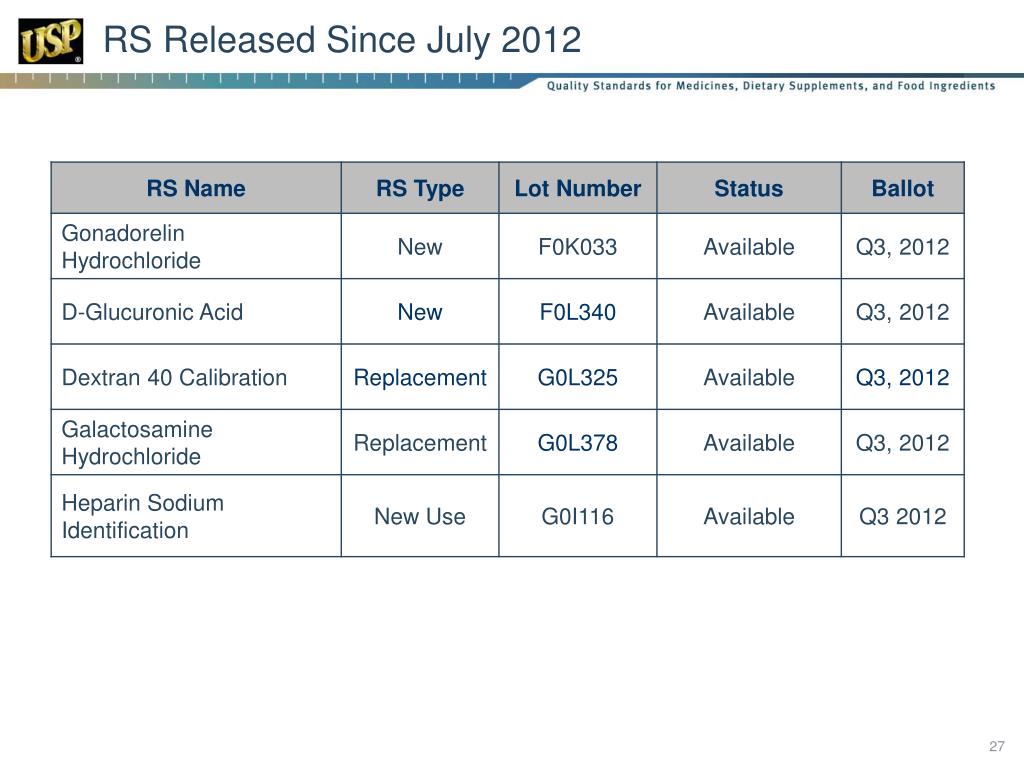
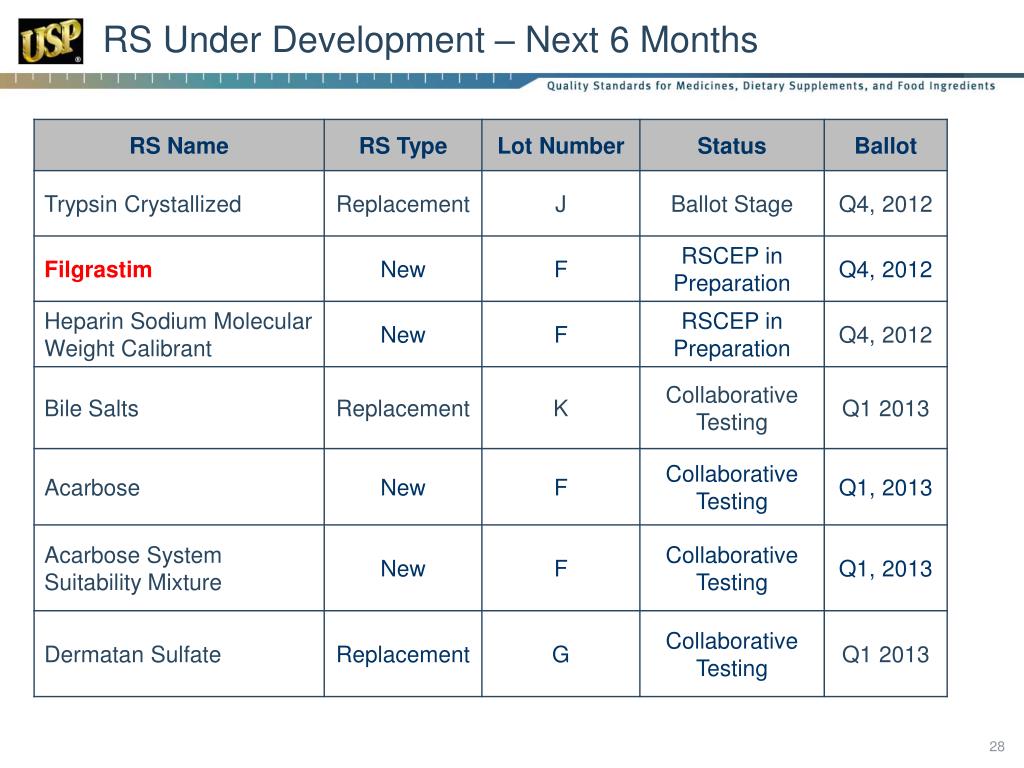
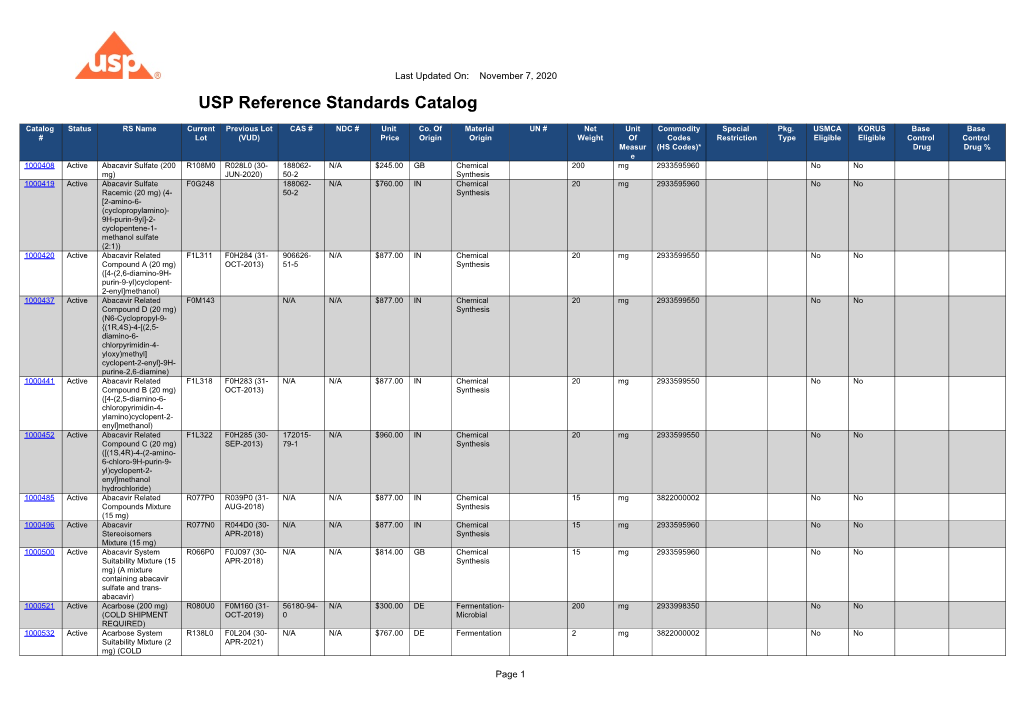
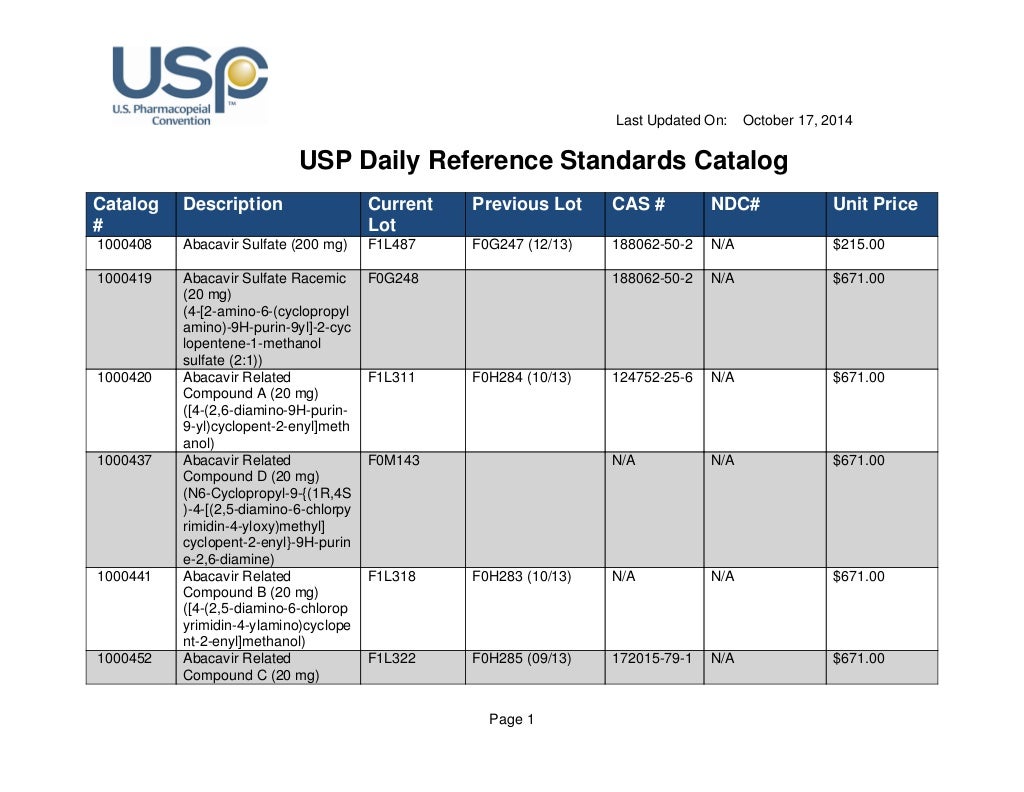
Usp Rs Catalogue
Usp Rs Catalogue - This product page in the online store can also be accessed by clicking on the catalog number of the rs in the usp reference standards catalog. Reference standards provided by the united states pharmacopeial convention (usp reference standards or usp rs) are highly characterized materials demonstrated to have the. See the new, updated, backordered, and validated lots of rs for april 2020. Find the latest information on usp reference standards (rs) for pharmaceutical substances and products. Find out the types, properties, and. Locally stocked usp rs ensure prompt delivery. Catalog # item description catalog # item description. Usp offers more than 3,500 reference standards for drug substances, excipients, food ingredients, impurities and more. If there is not a link to view the usp. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your compendial. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your compendial. Find the latest information on usp reference standards (rs) for the month of march 2020, including new, backordered, and changed lots. Locally stocked usp rs ensure prompt delivery. If there is not a link to view the usp. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) primary reference standards, ensuring you always have the right standards for your applications to. Visit the usp reference standards catalog and the online usp store for a complete listing of available usp rs's and to obtain rs documentation (e.g., usp certificates, sds), lot validity,. Catalog # item description catalog # item description. Find the latest information on usp reference standards (rs) for pharmaceutical substances and products. Find out how to access the official. 3500+ standards, including apis, impurities, excipients, & dietary supplements. Find out the types, properties, and. Usp offers more than 3,500 reference standards for drug substances, excipients, food ingredients, impurities and more. 3500+ standards, including apis, impurities, excipients, & dietary supplements. Quality solutions at a glance—navigate quickly through what’s new at usp —our most recently released usp reference standards (rs), pharmaceutical analytical impurities. Find out how to access the official. Catalog # item description catalog # item description. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Find out how to access the official. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your. Visit the usp reference standards catalog and the online usp store for a complete listing of available usp rs's and to obtain rs documentation (e.g., usp certificates, sds), lot validity,. Learn about the official usp reference standards (rs) for drugs, foods, and other substances, and how they are used in usp and nf monographs. Find out how to access the. Find the latest information on usp reference standards (rs) for pharmaceutical substances and products. Catalog # item description catalog # item description. If there is not a link to view the usp. Find out how to access the official. Find the latest information on usp reference standards (rs) for the month of may 2020, including new, backordered, and updated rs's. If there is not a link to view the usp. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your compendial. 3500+ standards, including apis, impurities, excipients, & dietary supplements. See the new, updated, backordered, and validated lots of rs for april 2020. Find out how to. Find out the types, properties, and. Reference standards provided by the united states pharmacopeial convention (usp reference standards or usp rs) are highly characterized materials demonstrated to have the. This product page in the online store can also be accessed by clicking on the catalog number of the rs in the usp reference standards catalog. We maintain a catalogue of. If there is not a link to view the usp. See the new, updated, backordered, and validated lots of rs for april 2020. Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Download the catalog, access the usp store, and explore. Find your usp authorized distributor and learn about new features and prices. Check the validity of rs lots and the online. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your compendial. Find the latest information on usp reference standards (rs) for the month of may 2020, including new, backordered, and updated rs's. Find out the types, properties, and.. Find the latest information on usp reference standards (rs) for pharmaceutical substances and products. If there is not a link to view the usp. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your compendial. Find out how to access the official. Catalog # item description catalog. Locally stocked usp rs ensure prompt delivery. Quality solutions at a glance—navigate quickly through what’s new at usp —our most recently released usp reference standards (rs), pharmaceutical analytical impurities. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: See the new, updated, backordered, and validated lots of. Learn about the selection, testing, and distribution of usp reference standards, which are substances used for pharmacopeial assays and tests. Find out how to access the official. Visit the usp reference standards catalog and the online usp store for a complete listing of available usp rs's and to obtain rs documentation (e.g., usp certificates, sds), lot validity,. Learn about the official usp reference standards (rs) for drugs, foods, and other substances, and how they are used in usp and nf monographs. Find the latest information on usp reference standards (rs) for the month of may 2020, including new, backordered, and updated rs's. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) reference standards, ensuring you always have the appropriate standard for your compendial. Locally stocked usp rs ensure prompt delivery. Usp offers more than 3,500 reference standards for drug substances, excipients, food ingredients, impurities and more. Find your usp authorized distributor and learn about new features and prices. Find the latest information on usp reference standards (rs) for pharmaceutical substances and products. Find out the types, properties, and. To help users of usp reference standards locate and apply materials relevant to their work, usp offers these convenient tools and resources: Empowering a healthy tomorrow excipient reference standards 012024 5/6 1547936 polysorbate 40 (2 g) 1547947. Download the catalog, access the usp store, and explore. Quality solutions at a glance—navigate quickly through what’s new at usp —our most recently released usp reference standards (rs), pharmaceutical analytical impurities. We maintain a catalogue of more than 3500 united states pharmacopeia (usp) primary reference standards, ensuring you always have the right standards for your applications to.PPT USP Reference Standards for Biologics PowerPoint Presentation
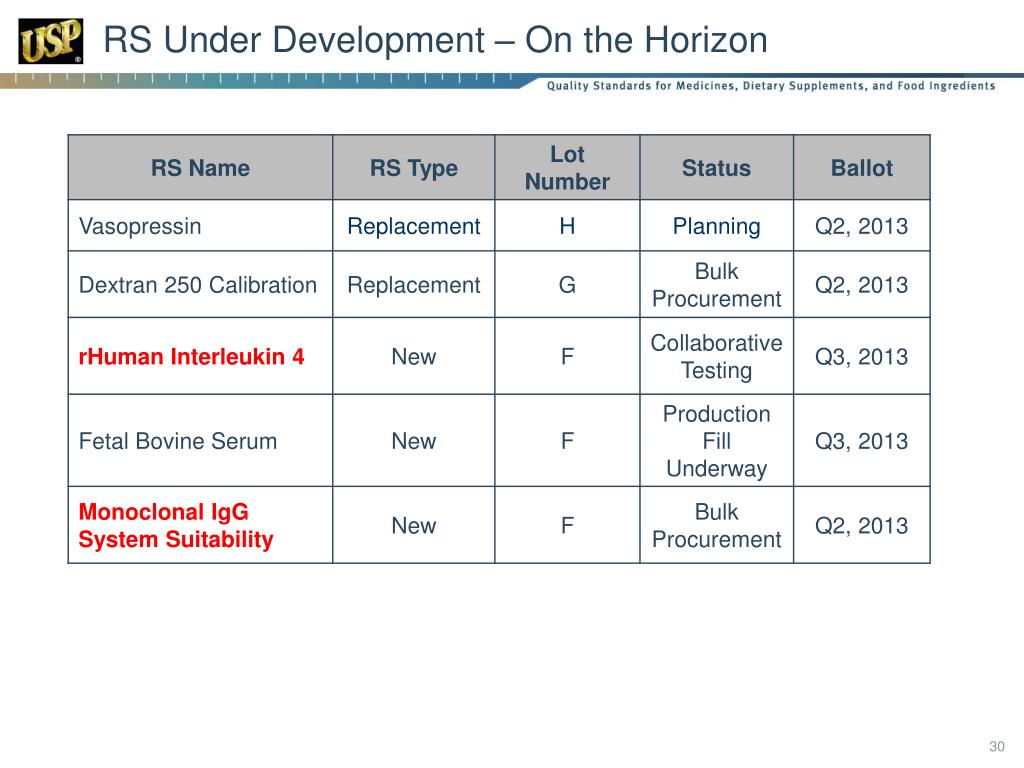
PPT USP Reference Standards for Biologics PowerPoint Presentation
USPStructuralConnectorsCatalogCanada.pdf Corrosion Zinc
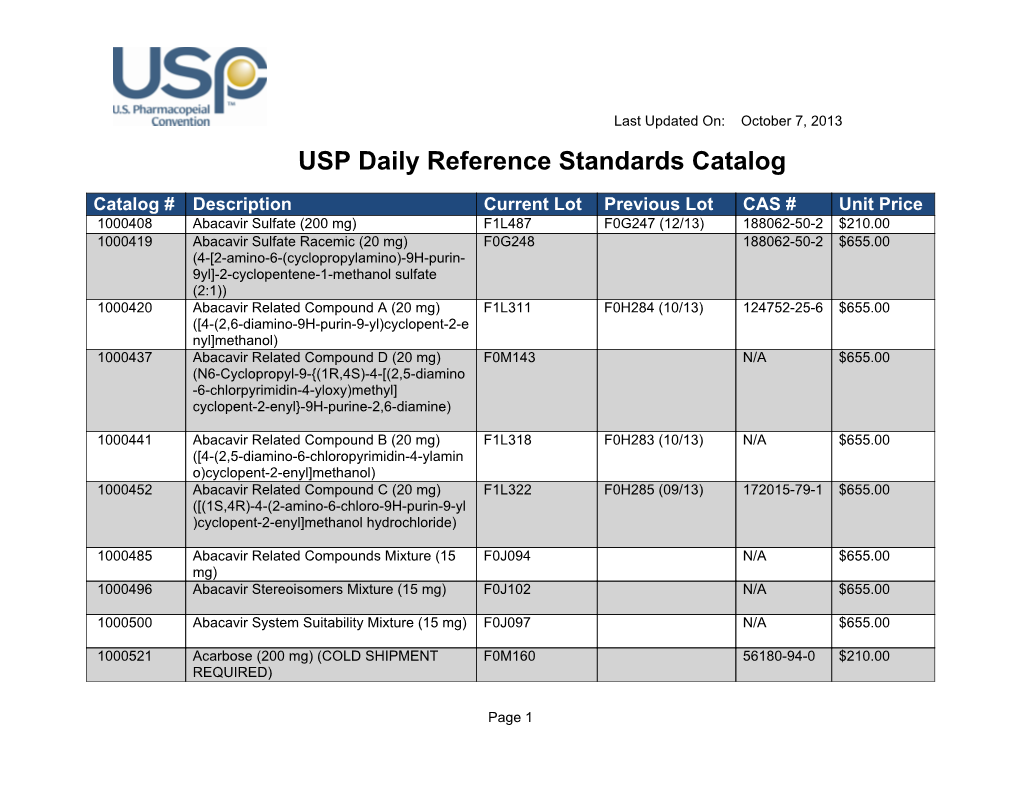
Usp daily reference standards catalog
USP Reference Standards Catalog DocsLib
USP Reference Standard AE Alchemist, Inc.
Usp daily reference standards catalog
Usp Reference Standards at 9999.00 INR in Ahmedabad, Gujarat Chemtech
PPT USP Reference Standards for Biologics PowerPoint Presentation
USP Daily Reference Standards Catalog DocsLib
Reference Standards Provided By The United States Pharmacopeial Convention (Usp Reference Standards Or Usp Rs) Are Highly Characterized Materials Demonstrated To Have The.
3500+ Standards, Including Apis, Impurities, Excipients, & Dietary Supplements.
If There Is Not A Link To View The Usp.
This Product Page In The Online Store Can Also Be Accessed By Clicking On The Catalog Number Of The Rs In The Usp Reference Standards Catalog.
Related Post: